Life Sciences & Biology
Private and public research institutions, analysis laboratories and pharmaceutical production sites study living organisms in the context of research, clinical trials and production control using optical instruments and image analysis software.
A wide range of image analysis software for all your microscopic analyses
Microvision Instruments offers a wide range of turnkey solutions for your various analyses
Bacteriology
Microvision Instrument bacteriology solutions allow the analysis of biological fluids or tissues in order to isolate or characterize one or more bacteria that may be responsible for a pathology.
Cytopathology
Cytopathology analyzes spontaneously shed cells or cells collected by abrasion or fine needle aspiration to diagnose diseases.
Histology
Histological examination is the microscopic analysis of a histological section that studies the cellular mechanisms involved in inflammatory, genetic, degenerative, tumoral or other diseases, constituting an extremely valuable diagnostic and detection tool.
Immunology
The immunological assessment studies the human body's reactions to a pathogenic organism (virus, bacteria, fungus or protozoa).
Microbiology
Microbiology analyzes microorganisms and the activities that characterize them to understand living systems at the microscopic level.
Microvision Instruments solutions for
life sciences
simple to implement, modular and scalable: from image analysis software to complete real-time systems, the combination adapted to your specific needs is available
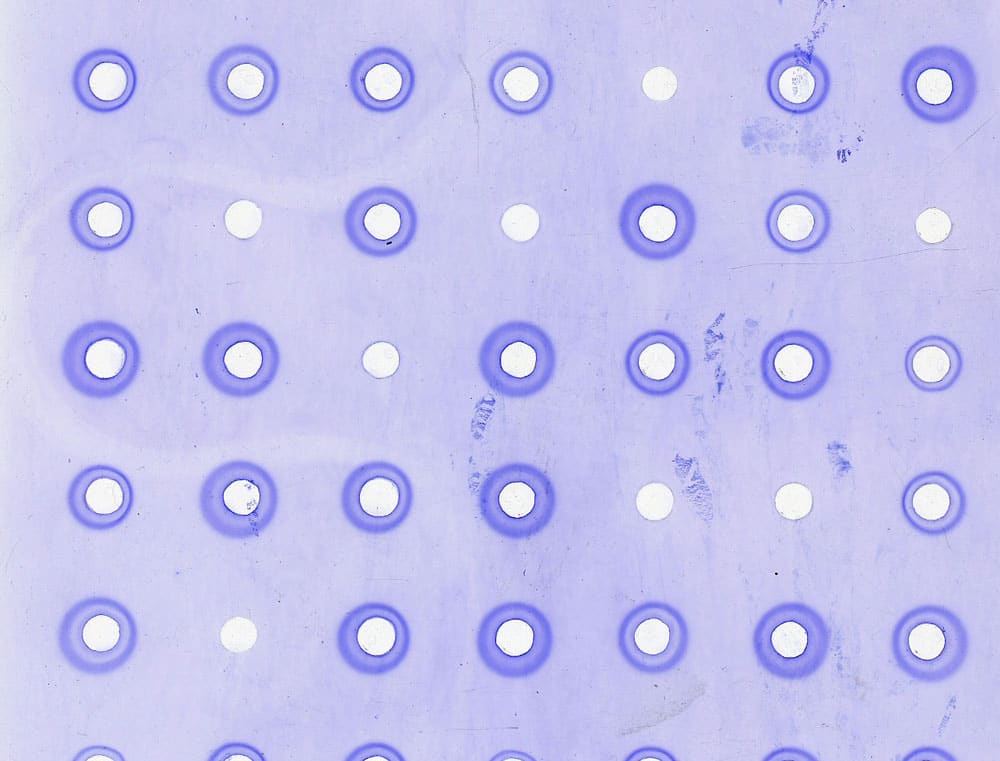


IMMULAB
Immulab analyzes your serological tests to control the dosing of serums using radial immunodiffusion and to measure antibiotic efficiency using antibiogram readings
- Serology
- Immunology
- Bacteriology
- FDA 21CFR Part 11 compliance
- Data Integrity compatibility
- Specific customizable standards
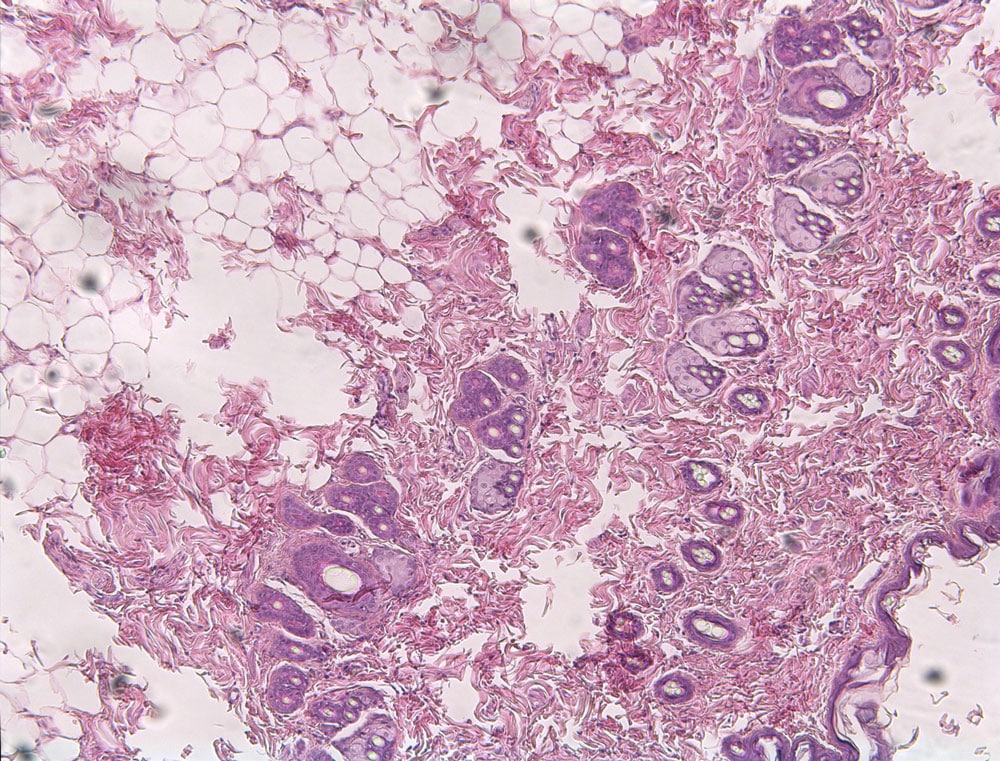


HISTOLAB
Automatic histology and morphometric measurements
- Histology / Morphometrics
- Colony counting
- Cell membrane monitoring
- Density
- Automatic measurement of tissue surface associated with a colouring
- Absorption and emission measurement
- FDA 21 CFR Part 11 compliance
- Data Integrity compatibility
- Specific customizable standards
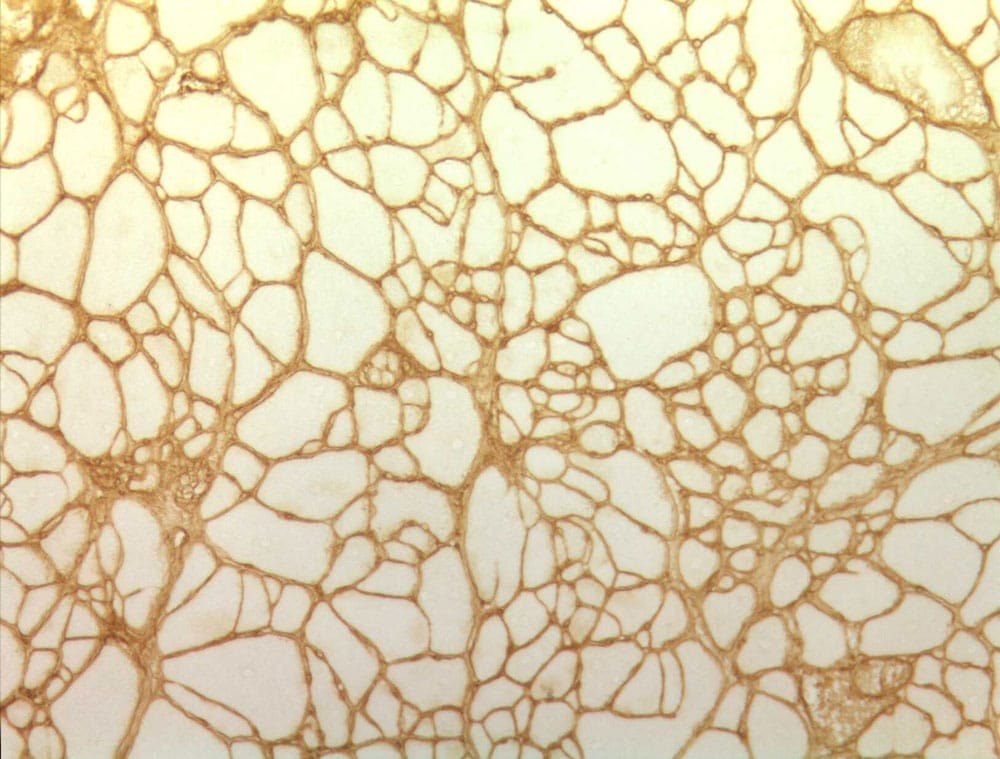


SAISAM
Aided morphometrics analysis: simultaneous measurement of several classes of objects
- Histology / Morphometrics
- FDA 21 CFR Part 11 compliance
- Data Integrity compatibility
- Specific customizable standards
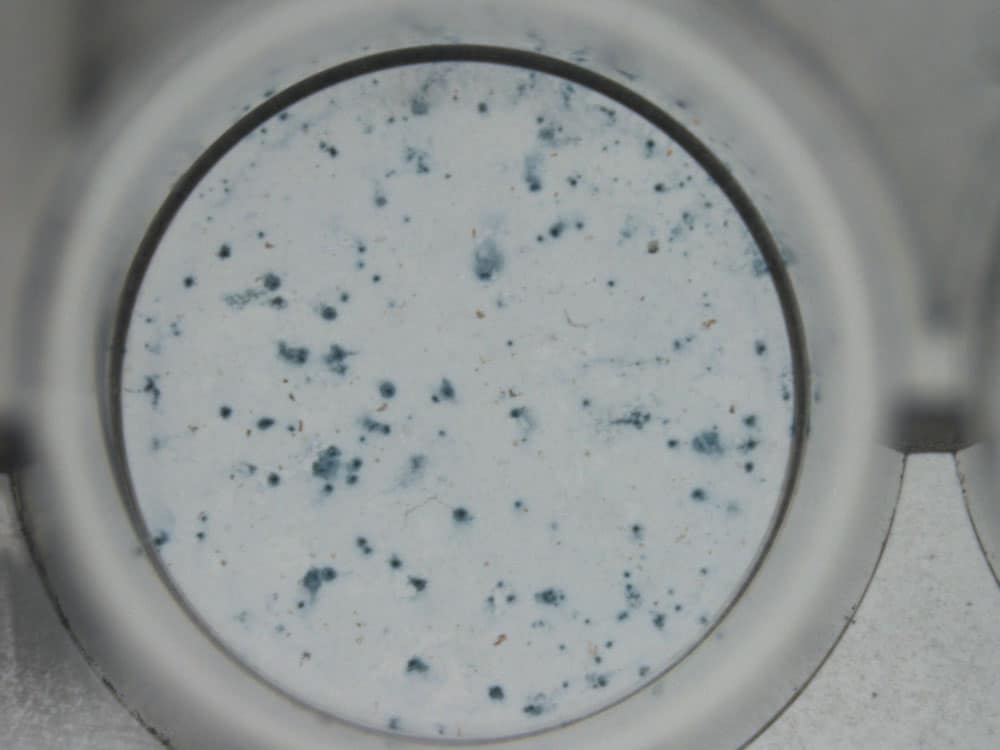


CELEST
CELEST is a cell cultures reader operating by means of viral range detection
- Virology / Immunology / Cytopathology
- FDA 21 CFR Part 11 compliance
- Data Integrity compatibility
- Specific customizable standards
Tell us more about your needs, we will put all our expertise at your service
Microvision Instruments is supporting you
Image acquisition hardware, image analysis software and service: together we build the effective and long-lasting solution that suits you

Optical instruments from leading optical manufacturers

Unique service offer to provide you a long-term support
La FAQ – Life science / Biology
What are the key features of image analysis software in pharmaceutical industry?
Image analysis software in pharmaceutical industry integrates several key features to facilitate various tasks within the industry. According to your needs, the software will include or be based on particle sizing and counting, particle shape analysis, texture and color analysis, image segmentation and thresholding, automated analysis and batch processing and reporting. Image analysis software in pharmaceutical industry are integrated to the laboratory equipment and offer user friendly interface and costumization to become a turnkey solution that complies with the industry regulation.